A state of matter represent various phases of matter that exhibit distinct properties based on the arrangement and behavior of particles.
How Many State of Matter Are There ?
Upto the latest advancement there are 7 states of matter namely
- 1. Solid
- 2. Liquid
- 3. Gas
- 4. Plasma
- 5. Bose-Einstein Condensate
- 6. Fermionic Condensate
- 7. Quark-Gluon Plasma
1. Solid
A state of matter characterized by a fixed shape and volume, where particles are closely packed and have limited movement.
Properties
Definite Shape and Volume:
Solids have a fixed shape and occupy a specific volume.
Strong Intermolecular Forces:
Particles in solids are closely packed and have strong attractive forces holding them together.
Limited Particle Movement:
The particles in solids have limited freedom of movement and vibrate around fixed positions.
High Density
Solids are generally more dense compared to liquids and gases due to the close packing of particles.
Low Compressibility
Solids have low compressibility, meaning they are resistant to changes in volume under pressure.
Read Also : Hypertime in Physics
Example: Ice is a solid form of water at low temperatures.
2. Liquid
A state of matter with a definite volume but no fixed shape, where particles can flow and move past one another.
Properties
Definite Volume, Indefinite Shape
Liquids have a fixed volume but take the shape of their container.
Moderate Intermolecular Forces
Particles in liquids have weaker intermolecular forces compared to solids, allowing them to flow past one another.
Moderate Particle Movement
The particles in liquids can move past one another, allowing the substance to flow.
Density Between Solids and Gases
Liquids have a density intermediate between that of solids and gases.
Low Compressibility
Like solids, liquids are also relatively incompressible.
Read Also : Gravity light
Example: Water in its liquid state is commonly encountered in everyday life.
3. Gas
A state of matter with no fixed shape or volume, where particles are widely spaced and move freely.
Properties
No Definite Shape or Volume
Gases have no fixed shape or volume and completely fill the container they are in.
Weak Intermolecular Forces
The particles in gases have very weak intermolecular forces, allowing them to move freely.
High Particle Movement
Gas particles move rapidly and in random directions, exhibiting high kinetic energy.
Low Density
Gases are less dense compared to solids and liquids due to the large separation between particles.
High Compressibility
Gases are highly compressible, meaning they can be easily compressed or expanded under pressure.
Example: The air we breathe is composed of gases like oxygen, nitrogen, and carbon dioxide.
4. Plasma
A highly energetic state of matter in which electrons are stripped from atoms, creating a mixture of charged particles.
Properties
Ionized Particles
Plasma consists of positively charged ions and free electrons due to the high energy levels.
No Definite Shape or Volume
Similar to gases, plasmas do not have a fixed shape or volume.
Electrically Conductive
Plasmas can conduct electricity due to the presence of free charged particles.
Reactiveness to Magnetic Fields
Plasmas are influenced by magnetic fields, exhibiting behaviors distinct from neutral gases.
High Energy State
Plasmas are characterized by high temperatures and are often found in extremely hot environments like stars.
Read Also : Electronic Waste
Example: The Sun and other stars are composed of plasma.
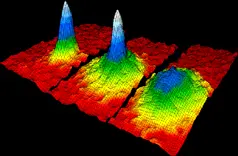
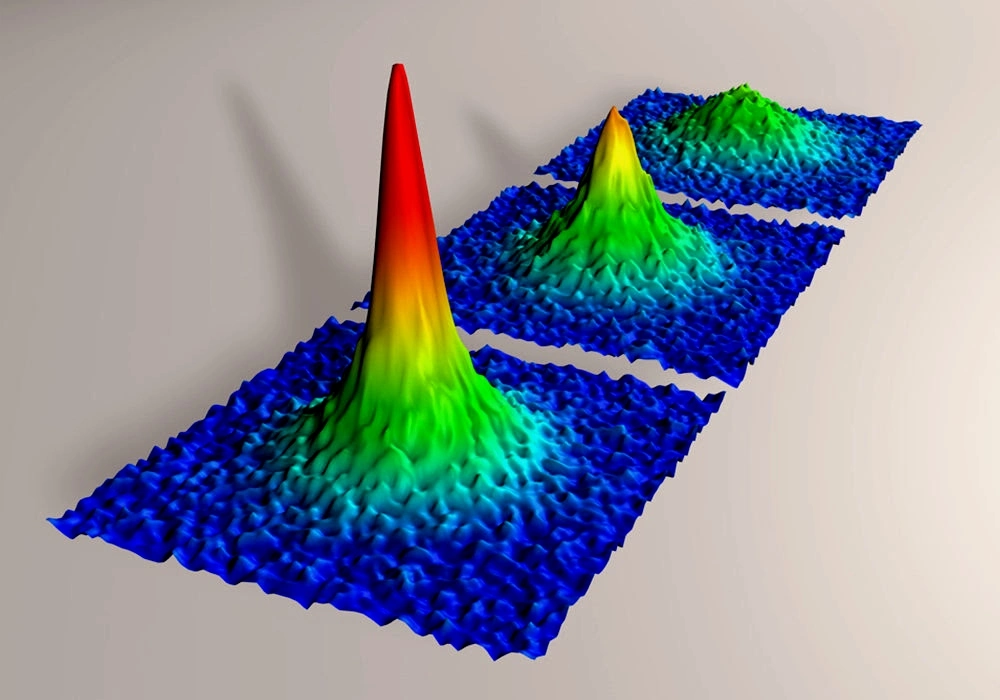
5. Bose-Einstein Condensate ( BEC)
A state of matter that occurs at extremely low temperatures, where a group of atoms behaves as a single quantum entity.
Properties
Superfluidity
BECs can flow without any viscosity, displaying unique fluid-like behavior.
Coherent Behavior
At low temperatures, particles in a BEC behave as a single quantum entity with synchronized states.
Ultralow Temperatures Required
Achieving a BEC requires cooling particles to extremely low temperatures near absolute zero.
Macroscopic Quantum Effects
BECs exhibit quantum mechanical behavior on a macroscopic scale.
Exotic Quantum Phenomena
BECs are used to study phenomena like interference patterns and quantum entanglement.
Example : This state has been created with ultra-cold atoms in laboratory settings.
6. Fermionic Condensate
A state of matter formed by fermions (particles with half-integer spins) at very low temperatures, exhibiting quantum effects on a macroscopic scale.
Properties
Fermionic Statistics
Particles in a fermionic condensate obey the Pauli exclusion principle, preventing multiple particles from occupying the same quantum state.
Low Temperatures Required
Creating a fermionic condensate necessitates cooling fermions to extremely low temperatures.
Quantum Degeneracy Pressure
Similar to a white dwarf or neutron star, fermionic condensates are stabilized by quantum degeneracy pressure.
Macroscopic Quantum Behavior
Fermionic condensates exhibit quantum mechanical effects on a macroscopic scale.
Exotic Quantum Phenomena
These condensates are used to explore phenomena like superfluidity and quantum phase transitions.
Example: Fermionic condensates have been produced using certain isotopes of helium.
7. Quark-Gluon Plasma
A state of matter thought to have existed in the early universe, composed of free quarks and gluons, which are the fundamental particles and carriers of the strong force.
Properties
Deconfined Quarks and Gluons
In QGP, quarks and gluons are no longer bound within hadrons (protons, neutrons), but exist freely.
Extreme Temperatures and Energies
QGP is created in high-energy collisions, reaching temperatures in the trillions of degrees Celsius.
Short-lived and Highly Energetic
QGP exists for an extremely short duration and requires specialized conditions to be observed.
Early Universe Conditions
It is believed to have existed in the first moments after the Big Bang, before hadrons formed.
High Energy Density
QGP has an extremely high energy density, surpassing that of nuclear matter.
Example : It is recreated in high-energy particle collisions, such as those that occur in particle accelerators like the Large Hadron Collider.
States of Matter NEET Questions
Here are few multiple-choice questions (MCQs) on the topic of States of Matter for NEET exam preparation:
The answer is also provided at the end
**Question 1:**
Which of the following is a characteristic property of a solid?
a) Indefinite shape
b) Definite volume
c) Highly compressible
d) Low intermolecular forces
**Question 2:**
What happens to the intermolecular forces as a substance transitions from a solid to a liquid state?
a) They weaken
b) They strengthen
c) They remain the same
d) They become zero
**Question 3:**
In which state of matter do particles have the highest degree of freedom of movement?
a) Solid
b) Liquid
c) Gas
d) Plasma
**Question 4:**
Which of the following statements about plasma is true?
a) It has a fixed shape
b) It is a low-energy state
c) It is electrically neutral
d) It is composed of charged particles
**Question 5:**
Which state of matter is characterized by particles behaving as both a wave and a particle?
a) Bose-Einstein Condensate
b) Fermionic Condensate
c) Quark-Gluon Plasma
d) Solid
**Question 6:**
What is the primary factor that determines whether a substance will be in a solid, liquid, or gaseous state at a given temperature and pressure?
a) Density
b) Atomic number
c) Intermolecular forces
d) Atomic mass
**Question 7:**
Which state of matter is known to exhibit superfluidity?
a) Liquid
b) Solid
c) Bose-Einstein Condensate
d) Gas
**Question 8:**
What happens to the volume of a gas when pressure is increased at constant temperature?
a) It increases
b) It decreases
c) It remains the same
d) It becomes zero
**Question 9:**
At what temperature does a Bose-Einstein Condensate typically form?
a) Room temperature
b) Just above absolute zero
c) Boiling point of water
d) Freezing point of water
**Question 10:**
Which state of matter exhibits the property of quantum degeneracy pressure?
a) Liquid
b) Solid
c) Fermionic Condensate
d) Gas
**Question 11:**
In which state of matter do particles have the highest kinetic energy?
a) Solid
b) Liquid
c) Gas
d) Bose-Einstein Condensate
**Question 12:**
Which state of matter can be influenced by magnetic fields?
a) Solid
b) Liquid
c) Gas
d) Plasma
**Question 13:**
What happens to the density of a gas when it is compressed?
a) Increases
b) Decreases
c) Remains constant
d) Becomes zero
**Question 14:**
What is the defining characteristic of a fermionic condensate?
a) It consists of fermions
b) It is a high-energy state
c) It is found at high temperatures
d) It is composed of charged particles
**Question 15:**
What type of particles make up a quark-gluon plasma?
a) Quarks and gluons
b) Electrons and protons
c) Neutrons and protons
d) Neutrons and electrons
**Question 16:**
Which state of matter is often found in stars?
a) Solid
b) Liquid
c) Gas
d) Plasma
**Question 17:**
What is the primary factor that distinguishes a Bose-Einstein Condensate from other states of matter?
a) Temperature
b) Density
c) Particle composition
d) Quantum behavior
**Question 18:**
At what temperature does a fermionic condensate typically form?
a) Absolute zero
b) Room temperature
c) Boiling point of water
d) Freezing point of water
**Question 19:**
Which state of matter is characterized by a highly ordered arrangement of particles?
a) Liquid
b) Solid
c) Gas
d) Plasma
**Question 20:**
What is the primary difference between a gas and a plasma?
a) Density of particles
b) Degree of particle movement
c) Presence of charged particles
d) Compressibility
Answers :
1. b) Definite volume
2. a) They weaken
3. c) Gas
4. d) It is composed of charged particles
5. c) Quark-Gluon Plasma
6. c) Intermolecular forces
7. c) Bose-Einstein Condensate
8. b) It decreases
9. b) Just above absolute zero
10. c) Fermionic Condensate
11. c) Gas
12. d) Plasma
13. a) Increases
14. a) It consists of fermions
15. a) Quarks and gluons
16. d) Plasma
17. d) Quantum behavior
18. a) Absolute zero
19. b) Solid
20. c) Presence of charged particles